Calorie restriction (CR), as a non-pharmacological dietary intervention, promotes health and extends lifespan in various organisms. It works by inducing a series of metabolic changes, but the specific metabolites responsible for these physiological benefits remain unclear. AMP-activated protein kinase (AMPK) plays a key role in the beneficial effects of CR and is an important target for identifying calorie restriction mimetics (CRMs). This study aims to identify the metabolites whose abundance changes during CR through metabolomics analysis, determine their functions, and explore whether specific metabolites can mimic the effects of CR.

1. Cell experiments: Mouse serum (CR serum) from 4-month-old mice was used to treat mouse embryonic fibroblasts (MEFs), HEK293T cells, primary hepatocytes, and primary myocytes. Immunoblotting was used to detect AMPK activation. The effects of heat treatment, dialysis, or Lipidex column treatment of CR serum on AMPK activation were observed. Polar metabolites that increased after CR were screened and tested for their ability to activate AMPK.
2. Animal experiments: Mice were subjected to CR, fasting, and cardiotoxin treatment. Fecal microbiota transplantation experiments were conducted by orally administering different treatments of feces to mice. (2-Hydroxypropyl)-β-cyclodextrin-coated LCA was used to treat mice, and the concentration of LCA in serum and tissues, AMPK activation, and aging-related phenotypic changes were measured. The effects of LCA on the lifespan and healthspan of nematodes and fruit flies were studied.
Detection methods: Various mass spectrometry techniques were used to analyze metabolites in serum, tissues, and cells. Immunoblotting was used to detect protein expression and phosphorylation levels. Quantitative magnetic resonance was used to measure mouse body composition. A metabolic cage system measured the energy expenditure of mice. Histological staining and immunohistochemistry were used to observe tissue morphology and protein expression. qPCR was used to measure mRNA levels. Transmission electron microscopy was used to observe mitochondrial morphology, etc.

Figure 1: CR-treated mouse serum activates AMPK in cells and mice
1.1 Figures 1a – 1d: After 4 hours of treatment with CR serum, MEFs, HEK293T cells, primary hepatocytes, and primary myocytes were analyzed by immunoblotting for pAMPKα and pACC levels. It was found that CR serum can activate AMPK in these cells, indicating that CR serum can simulate some of the effects of CR at the cellular level.
1.2 Figures 1e – 1f: After 2 hours of CR serum infusion in mice with free access to food, AMPK activation was detected in liver and muscle tissues. The results showed that CR serum could activate AMPK in mice, demonstrating its ability to activate AMPK at the organismal level.
1.3 Figures 1g – 1i: After different treatments of CR serum, it was found that heat-treated CR serum could still activate AMPK, while dialyzed CR serum lost this ability. Moreover, after treatment with Lipidex columns, the eluted fraction could still activate AMPK, but with reduced capacity. This suggests that heat-stable, low-molecular-weight polar metabolites in CR serum mediate AMPK activation, and non-polar compounds and lipids likely do not participate in this process.
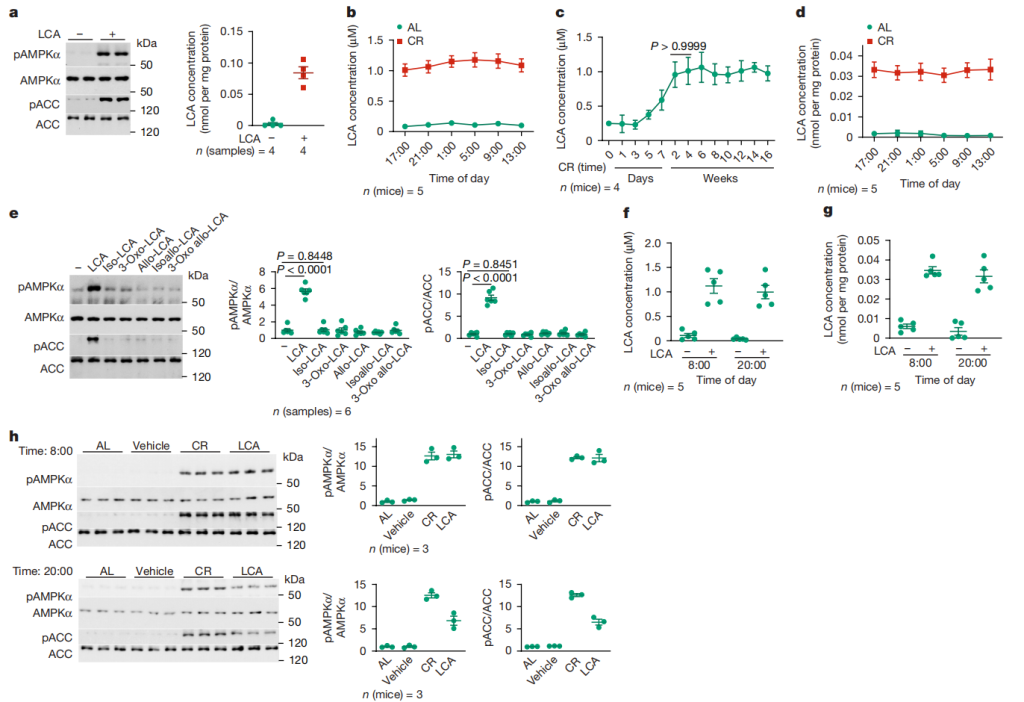
Figure 2: LCA increases after CR and is responsible for activating AMPK
2.1 Figure 2a: After 4 hours of 1μM LCA treatment of MEFs, AMPK activity and intracellular LCA concentration were measured. The results indicated that LCA is the factor in CR serum that activates AMPK, and the intracellular LCA concentration after treatment was similar to the concentration in CR mouse tissues.
2.2 Figures 2b – 2d: Metabolomics analysis showed that the concentration of LCA in serum and muscles of mice treated with CR for 4 months significantly increased at various time points and with CR duration.
2.3 Figure 2e: After 4 hours of treatment with 1μM LCA or its derivatives, AMPK activity was measured. It was found that only LCA activated AMPK, while other bile acids such as CA and CDCA did not.
2.4 Figures 2f – 2g: After 1 month of drinking water containing (2-hydroxypropyl)-β-cyclodextrin-coated LCA, serum and muscle tissue LCA concentrations were measured. It was found that the LCA levels were similar to those in CR serum.
2.5 Figure 2h: After giving elderly mice either LCA-containing water or CR treatment, AMPK activation in skeletal muscles was measured. The results indicated that LCA could activate AMPK in mice, with effects similar to CR.
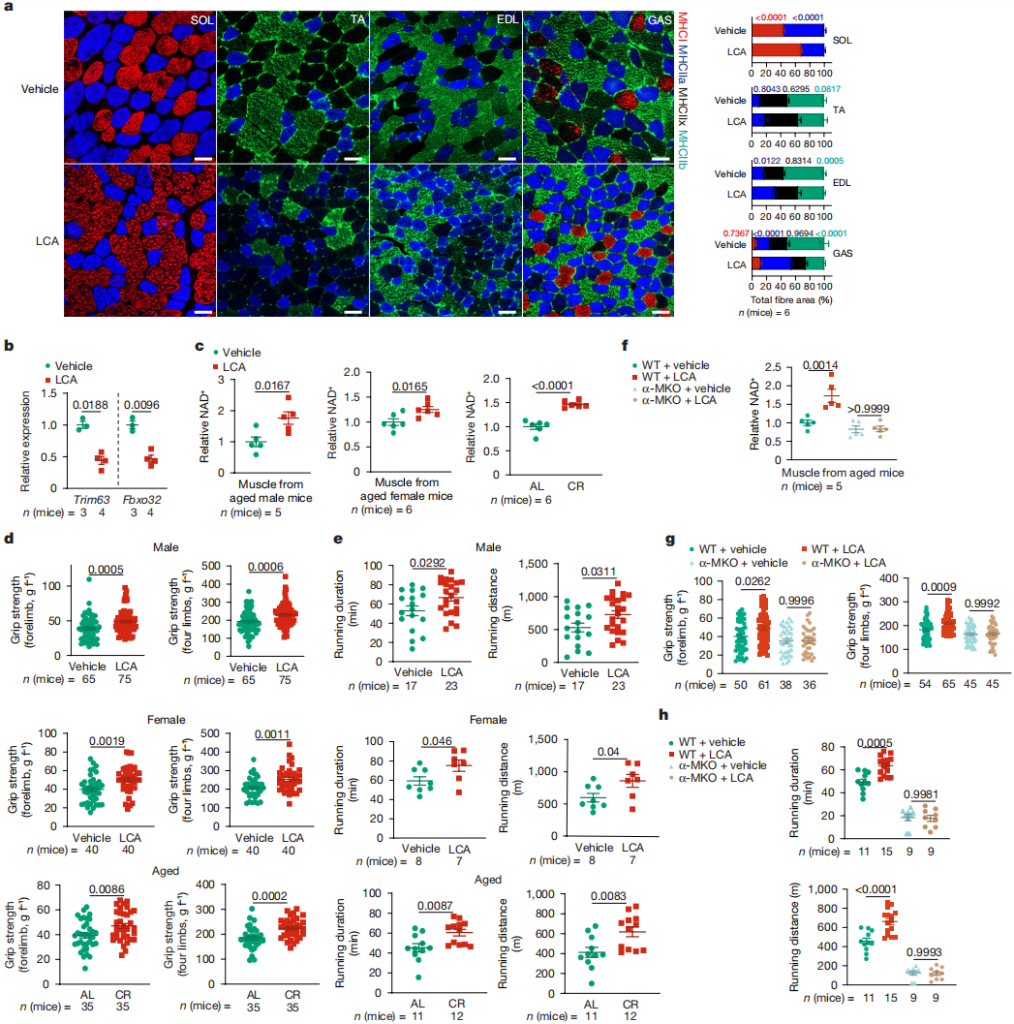
Figure 3: The rejuvenating effect of LCA depends on AMPK
3.1 Figures 3a – 3b: After 1 month of drinking water containing (2-hydroxypropyl)-β-cyclodextrin-coated LCA, muscle fiber types were detected by immunohistochemistry, and mRNA levels of atrophy markers Trim63 and Fbxo32 were measured by RT-PCR. It was found that LCA induced oxidative fiber conversion and prevented muscle atrophy.
3.2 Figure 3c: NAD⁺ levels in muscle tissue of elderly mice were measured, and it was found that LCA treatment increased NAD⁺ to levels similar to those induced by CR.
3.3 Figures 3d – 3e: Grip strength and endurance were measured in elderly mice, and it was found that LCA promoted muscle strength and endurance, with effects similar to CR.
3.4 Figures 3f – 3h: After knocking out muscle-specific AMPKα, the effects of LCA on increasing NAD⁺ levels, muscle strength, and endurance were eliminated, suggesting that AMPK is essential for LCA’s action.

Figure 4: LCA extends lifespan and healthspan
4.1 Figures 4a – 4b: Treatment of wild-type (N2) or aak-2 knockout nematodes and wild-type (Act5C-GAL4) or AMPKα knockdown fruit flies with 100μM LCA showed that LCA could extend the lifespan of nematodes and fruit flies by activating AMPK.
4.2 Figure 4c: Nematode pumping rate was measured, and it was found that LCA could promote an increase, and this effect depended on AMPK.
4.3 Figures 4d – 4e: After treating nematodes and fruit flies with LCA and then inducing oxidative stress with FeSO₄ and paraquat, it was found that LCA promoted oxidative stress resistance in both organisms, and this effect depended on AMPK.
4.4 Figure 4f: NAD⁺ levels in nematodes and fruit flies were measured, and it was found that LCA increased these levels, and this effect depended on AMPK.
4.5 Figure 4g: After giving 1-year-old wild-type mice water containing (2-hydroxypropyl)-β-cyclodextrin-coated LCA, lifespan was measured, and the results showed a trend toward increased median lifespan in LCA-treated mice.
It is worth mentioning that the QMR06-090H body composition analyzer from Suzhou Newmai Analytical Instruments Co., Ltd., can be used to measure lean body mass and fat body mass in aged mice after LCA treatment. Compared to the control group, the clear display of LCA treatment effects, such as preventing muscle atrophy and adjusting the fat-to-body-weight ratio, provides crucial quantifiable data for studying the efficacy of LCA. The key findings from the body composition measurements are as follows:
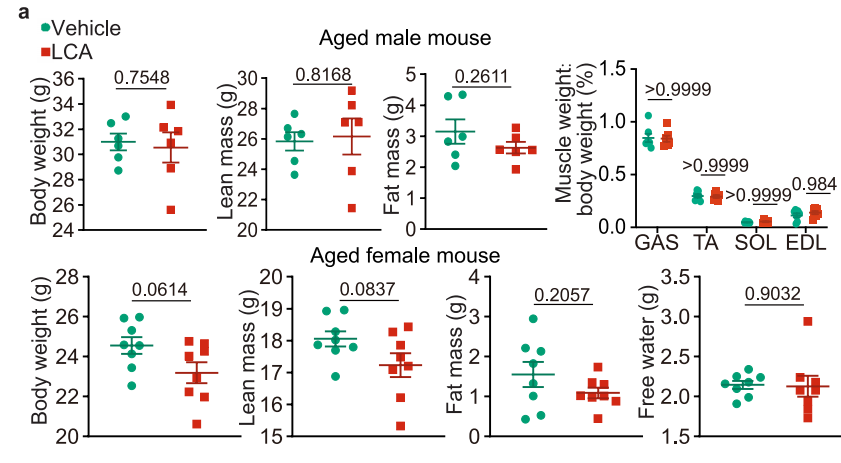
Figure 5: LCA improves muscle function in aged mice
5.1 Figure 5a: LCA prevents muscle atrophy in aged mice: After 1 month of feeding male and female aged mice (2-hydroxypropyl)-β-cyclodextrin-coated LCA (1g/L in drinking water), LCA-treated mice showed a significant trend of maintaining or increasing muscle mass compared to the control group. This suggests that LCA effectively prevents muscle atrophy due to aging in aged mice and has a positive protective effect on muscle health. In addition to muscle mass, data from this analyzer also suggests that LCA treatment might affect fat body mass and overall body weight in mice. Although the paper does not discuss changes in fat body mass and weight in detail, the comprehensive data from body composition measurements suggest that LCA may influence fat accumulation or depletion through some metabolic regulation mechanism, thereby adjusting the fat-to-lean body mass ratio and maintaining the balance of body composition.
This study shows that LCA is a metabolite induced by CR, which can simulate CR’s anti-aging effects through an AMPK-dependent pathway, providing a new direction for developing interventions that improve healthspan. However, the study also has limitations, such as the possibility of other unidentified metabolites that activate AMPK, the need for further research on the significance of LCA’s lifespan-extending effects in mice, and the necessity of further investigation into the differences in LCA’s mechanisms across species. Future research could focus on these areas to better understand LCA’s effects and application potential.

Qu, Q., Chen, Y., Wang, Y. et al. Author Correction: Lithocholic acid phenocopies anti-ageing effects of calorie restriction. Nature 638, E6 (2025). https://doi.org/10.1038/s41586-025-08693-w
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top