Hydrophilicity refers to a molecule’s ability to attract and interact with water, often due to the presence of polar functional groups. These molecules readily absorb or dissolve in water. Solid materials composed of such molecules tend to have surfaces that are easily wetted by water, which characterizes their hydrophilic nature.
Hydrophobicity describes molecules that are typically non-polar and preferentially dissolve in neutral or non-polar solvents (such as organic solvents). In water, hydrophobic molecules tend to cluster together. On hydrophobic surfaces, water tends to form droplets with large contact angles, indicating poor wetting behavior.
The wetting behavior of a material’s surface is essentially a result of interfacial energy changes. When the cohesive forces between water molecules are weaker than the adhesive forces between water and the solid surface, the material becomes wetted—this is a hydrophilic material. Conversely, if the cohesive forces between water molecules are stronger, the water cannot effectively wet the surface, and the material is considered hydrophobic (or water-repellent).

In aqueous environments, particles may aggregate through processes such as coagulation, selective agglomeration, hydrophobic aggregation, or oil bridging. These mechanisms have found wide industrial use in mineral processing, water treatment, and food processing. In contrast, the reverse process—particle dispersion—is critical in industries such as powder technology, chemical engineering, coatings, and pharmaceuticals, where it improves processing efficiency and enhances product quality and performance.
The hydrophilic or hydrophobic nature of a material is directly related to the aggregation or dispersion of particles. Low-field nuclear magnetic resonance (LF-NMR) technology enables the study of how particles disperse in water and how this behavior is influenced by surface wettability. By analyzing the interactions between particles, we can gain insights into the mechanisms of dispersion.
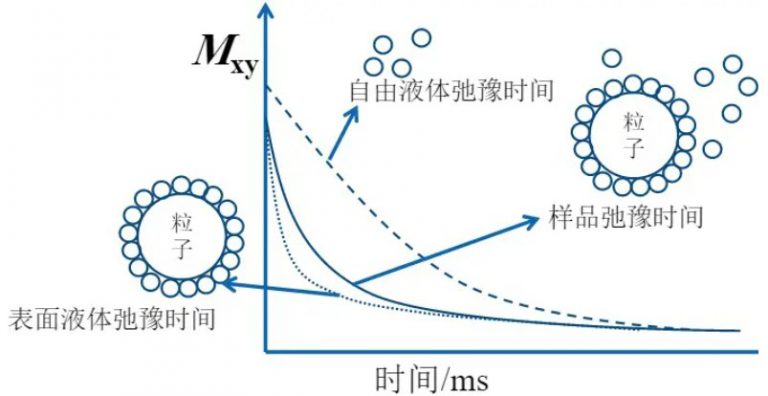
In particle dispersions, the relaxation rate of the solvent is linearly proportional to the available surface area of the particles. Solvents associated with free polymers or those trapped within polymer loops and tails typically show little change in relaxation rate due to their high mobility. However, when polymers form an adsorption layer on the particle surface, the local concentration and/or residence time of water molecules near the surface increases, leading to a noticeable enhancement in the overall relaxation rate. This relaxation difference, measured via LF-NMR, can be used to characterize particle dispersion behavior.

Nuomag PQ001 Series Low-Field NMR System
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top