Polymorphism is widespread among solid compounds. For medicines, polymorphism can affect product quality and therapeutic outcomes, which is why it has attracted growing attention worldwide. Today, polymorph studies are an essential part of new drug discovery and regulatory submission. Crystal-form research plays a pivotal role in optimising developability, lowering risk, ensuring quality, and building robust patent protection—often proving decisive for success or failure.
Solid drugs commonly exist as multiple forms—polymorphs, solvates, co-crystals, etc. Broadly, polymorphism refers to a substance existing in two or more distinct crystal lattices. Solid forms are generally classified as crystalline or amorphous, depending on whether internal constituents (atoms, ions, molecules) are ordered or disordered. Different crystal forms can alter dissolution and absorption rates in vivo, thereby impacting bioavailability, clinical efficacy, and safety.
By stability, polymorphs are typically classified as stable, metastable, and unstable. Stable forms often have lower entropy, higher melting points, and better chemical stability—but also lower solubility and dissolution rates, leading to poorer bioavailability. Unstable forms are the opposite. Metastable forms tend to transform into stable forms during storage. Because crystal form dictates properties such as melting point, density, hardness, morphology, and formulation stability, differences in free energy and intermolecular interactions between polymorphs result in distinct solubility, dissolution, and bioavailability, ultimately altering absorption rate and therapeutic effect.
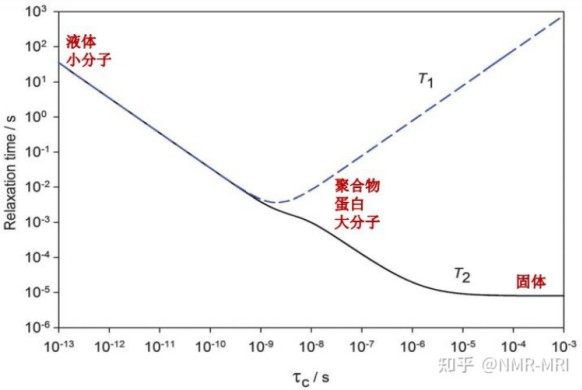
Crystalline and amorphous APIs exhibit markedly different T1 relaxation behaviour, making T1 a powerful discriminator. Typically, crystalline forms show longer T1 than amorphous forms. Because relaxation times relate to rotational correlation times, they reflect molecular mobility: in solids, lower mobility generally means longer T1. Time-domain NMR thus provides an effective means to assess crystallinity in API powders.
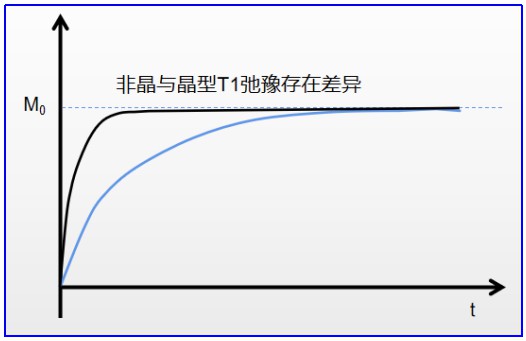
At a fixed temperature, a drug with a stable crystal form exhibits a nearly constant T1. Upon polymorphic transformation, the measured T1 changes accordingly. By tracking T1 behaviour, time-domain NMR can monitor crystalline content in physical mixtures and follow polymorphic transitions in real time.
The 0.5 T benchtop permanent-magnet NMR analyser PQ001 is a cost-effective choice for laboratories, offering an economical solution for crystal-form assessment.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top