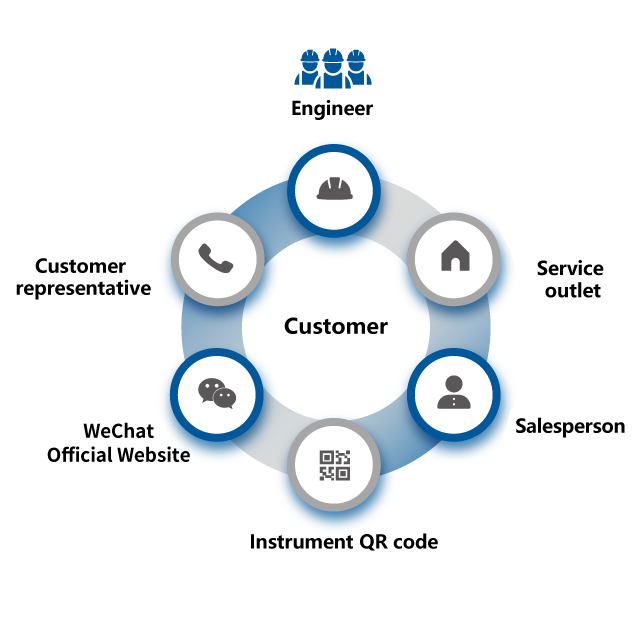
Service Hotline

Training Services
Maintenance & Value-Added Services

Online Support

Consumables Supply

Shared Knowledge Base

Application Solutions
● Before formal testing, the magnet temperature control of the NMR system must reach and stabilize within ±0.03℃ of the preset value. The typical preset is 32℃ (35℃ for the cross-link density analyzer);
● All other switches on the cabinet should be ON with indicator lights illuminated;
● Software launches normally;
● Use the calibration oil sample with the FID sequence to complete SF calibration and P1/P2 calibration.
● Before NMR testing, no complex pretreatment is required. Solid samples should be cut to fit the holder, then weighed;
● Liquid samples must be sealed, e.g., bagged or bottled in capped glass vials;
● For vertical holders, the sample height should not exceed 2.5 cm and is not recommended below 1 cm;
● For horizontal holders, the sample length should not exceed 3 cm (for 60 mm bore systems, avoid lengths over 5 cm).
● First check whether the monitor cable is loose;
● If secure and still not working, reseat the RAM and interface boards in the PC;
● Possible poor contact;
● Open the NMR system’s PC chassis, locate the boards, and reseat them;
● Locate the RAM module(s) and reseat them;
● Do not use excessive force when reseating.
● Confirm the RF unit is powered on;
● Ensure the probe contains the calibration sample;
● Verify SF and O1 match the factory report or a known-good configuration; adjust if they differ;
● Check rear-panel cabling for poor contacts; replace with a spare RF cable if needed.
In low-field NMR, suggested parameter presets:
1) Dry powders / dry samples / plastics:
T2: set TE to the minimum the instrument allows, i.e., slightly greater than (12*P1 + 6*P2) – P1 and P2 are shown in the left panel and vary by system; set NECH so the signal fully decays with a short flat tail; TW 500–2000 (tune per TW method); NS 16–64 depending on SNR (rule of thumb: highest initial point divided by the average of the last few points > 50); SW 200.
2) Fresh meat / seafood / fruit:
T2: TE 0.5–0.8 ms; NECH so the signal fully decays with a short tail (typically 10,000–18,000); TW 6000–8000; NS ≈ 4 depending on SNR; SW 100.
3) Gels:
T2: TE 0.4–0.6 ms; NECH to full decay with short tail (≈ 10,000–18,000); TW 8000–10,000; NS ≈ 4; SW 100.
4) Rubber:
T2: TE ≈ 0.2 ms; NECH to full decay with short tail; TW 500–3000; NS ≈ 8; SW 200.
5) Liquids:
Oils/fats: T2 TE 0.2–0.4 ms; NECH 4000–6000; TW 2000; NS 2–8 per SNR; SW 100.
Pure water (DI/ultrapure): T2 TE ≈ 1 ms; NECH 18,000; TW 18,000; NS ≈ 2; SW 100.
Ethanol: similar to water; slightly smaller TE/NECH/TW, e.g., TE 0.8 ms, NECH 15,000, TW 15,000.
● After acquisition, the CPMG decay should fully attenuate and level off at the end;
● The decay should be smooth and exponential with no point dropouts; if not fully decayed, increase NECH (up to ~18,000) or TE — prioritize increasing NECH;
● Check the vertical scale: higher initial amplitude improves SNR; typically aim for thousands to tens of thousands;
● If the signal is weak, increase NS.
● After acquisition, the IR recovery should complete with the last 2–3 points stable and no obvious upward trend; the curve should be smooth and exponential;
● If recovery is incomplete, increase TW and reset NTI;
● For better SNR, aim for a higher peak amplitude (typically > 100);
● If the signal is weak, increase PRG and NS as needed.
SEQ: sequence
SF: spectrometer frequency
O1: offset 1
P1: pulse 1 [90° pulse]
TD: time data
DR: data radius
PRG: pre-amp regulate gain
SW: sampling bandwidth
RFD: RF delay (regulate first data)
TW: recycle delay (time wait)
RG1: analog gain 1
DRG1: digital gain 1
NS: number of scans (sampling)
P2: pulse 2 [180° pulse]
TE: echo time
NECH: number of echoes
Peaks parity: peak picking
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top