Natural gas hydrates are widely distributed in subsea sediments and permafrost regions around the world. Beyond their abundant global reserves, the clean combustion characteristics and high energy density of natural gas hydrates make them one of the most promising alternative energy resources for the future. As a result, the demand for commercialising natural gas hydrates continues to grow, attracting significant attention from scientists and engineers worldwide[1].

Due to their occurrence in unconventional reservoirs such as subsea sediments, natural gas hydrates present significant exploration and development challenges. Researchers have long focused on formation mechanisms, reservoir properties, and production methods. Efficient and safe extraction of natural gas directly impacts commercial viability, while understanding the gas production behaviour and dynamic gas release characteristics during hydrate dissociation is essential.

This case study employs nuclear magnetic resonance (NMR) for in situ, real-time monitoring of the natural gas hydrate formation process, investigating the kinetic characteristics of methane hydrate formation in porous media[2].
The sample was loaded into a reaction vessel and placed inside the NMR instrument. The flow switch was turned on, and the initial temperature of the coolant was set to 10°C. The NMR system was then started, and the software automatically monitored the sample’s NMR signal.
Once the sample reached 10°C, the temperature was further lowered at a rate of 5°C/h. When it reached 0°C, the cooling rate was adjusted to 2°C/h. As the temperature dropped to -2°C, a rapid decline in signal intensity indicated that the water in the sample had frozen. Cooling was then stopped, maintaining a stable temperature at -2°C.
After the water in the sample had frozen, methane gas was introduced into the reactor at 8.5 MPa. The pressure was then held constant while the system remained unchanged.
After the water in the sample had frozen and pressure remained constant for a prolonged period, the temperature was increased at a rate of 0.02–0.05°C/min. Simultaneously, changes in NMR signal intensity and pressure were monitored.
When the pressure gauge indicated a drop in pressure, methane hydrate formation had begun, and the temperature was maintained at this level. The NMR signal stopped rising and gradually decreased. Finally, the pressure reached a lower stable value, and the NMR signal also stabilised, marking the completion of methane hydrate formation.
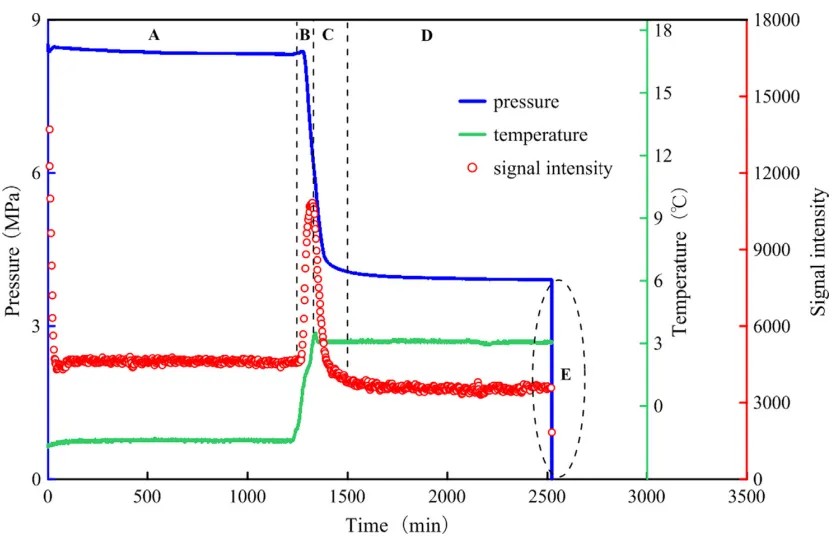
Figure 1: Relationship of pressure, temperature, and NMR signal intensity with time during hydrate formation
Using low-field NMR, this study monitored the in situ kinetics of methane hydrate formation, leading to the following conclusions:
The figure above illustrates the changes in NMR signal during hydrate formation, divided into four stages (A–D): induction, nucleation, growth, and stabilization.
A) Induction stage (0–1200 min): NMR signal rapidly decreases, indicating water in the sample has frozen;
B) Nucleation stage (1200–1324 min): As the temperature rises, frozen water gradually melts, NMR signal spikes, and pressure drops. Methane molecules occupy free water molecules to form the hydrate lattice.
C) Growth stage (1324–1500 min): After heating ceases, the NMR signal declines rapidly as more methane hydrate forms with falling pressure.
D) Stabilization stage (1500–2522 min): Temperature, pressure, and NMR signal remain relatively stable, indicating that methane hydrate formation is complete.
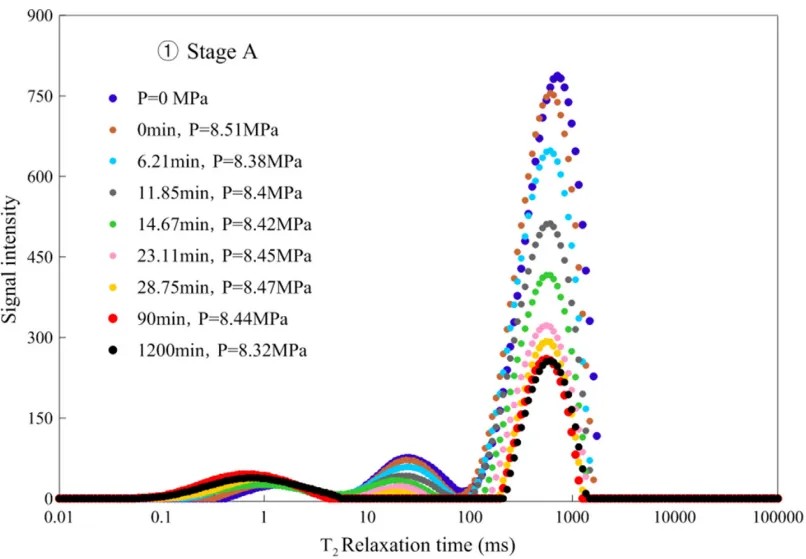
Figure 2: T2 relaxation time distribution during stage A of hydrate formation
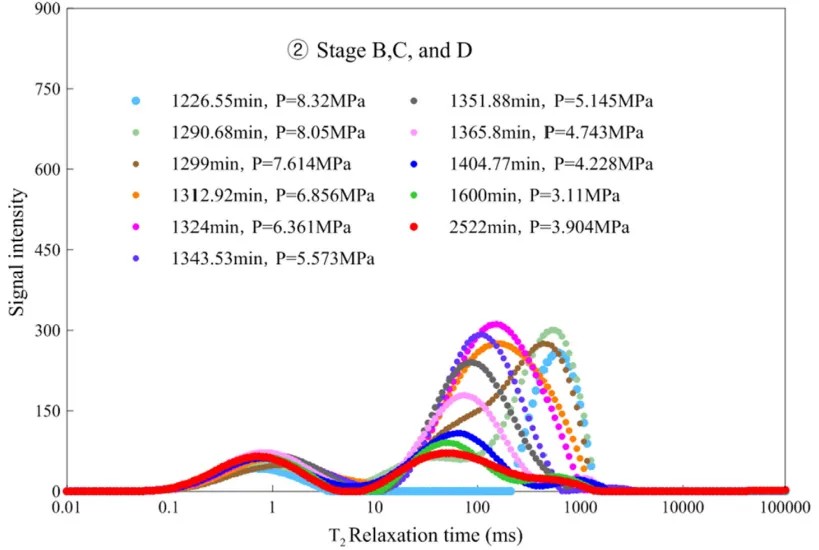
Figure 3: T2 relaxation time distribution during stages B, C, and D of hydrate formation
According to Figure 2, pore water is classified into three types: small pores (T2<9 ms), medium pores (9 ms<T2<100 ms), and large pores (T2>100 ms). Freezing begins in large pores and gradually progresses to smaller pores.
Figure 3 shows the T2 relaxation distribution of hydrate formation from nucleation to completion. At 1324 minutes (temperature 3.1°C, pressure 6.36 MPa), water signal peaks. As hydrate growth continues, the NMR signal declines. Large pore water decreases significantly, while remaining water exists in medium and small pores. Some large pore water migrates to smaller pores, whereas small pore water remains largely unchanged due to surface adsorption effects.
Low-field NMR not only enables real-time monitoring of hydrate formation, but also allows in situ tracking of dissociation processes, providing insight into methane gas release mechanisms and flow evolution in hydrate-bearing sediments. This information is critical for assessing sediment permeability and wettability, guiding the development of natural gas hydrate resources.
For inquiries regarding the above application, please contact: 15618820062
[1] Gainullin S E, Kazakova P Y, Pavelyev R S, et al. New Promoters Derived from Amino Acids and Citric Acid for the Efficient Storage of Methane As Gas Hydrates[J]. Chemistry and Technology of Fuels and Oils, 2024, 60(4):848-854.
[2] B, Jing Zhan A, et al. Experimental research on methane hydrate formation in porous media based on the low-field NMR technique [J]. Chemical Engineering Science, 244 (2021).
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top