Prussian Blue compounds (PBAs) are highly promising sodium-ion battery (SIB) cathode materials due to their remarkable advantages, including low cost, high specific capacity and energy density, excellent rate performance, and long cycle life[1].

However, PBAs synthesized in aqueous environments contain a certain amount of crystalline water, which can cause significant side effects during battery operation. Variations in crystalline water content can not only affect the material’s crystallinity but also influence charge transfer and energy storage performance, with substantial impact on calendar life and high-temperature storage stability.
The most common approach for managing crystalline water is thermal treatment. This method is simple and effective but still presents serious challenges. Samples treated thermally are prone to rehydration in air, and with increasing charge-discharge cycles, the crystalline water content gradually rises[2].
Conventional methods for detecting crystalline water in Prussian Blue compounds include drying and thermogravimetric analysis (TGA).
Typically, Prussian Blue samples are dried at 120°C, and the mass change is recorded until it stabilizes. Under this condition, the total mass of free water and adsorbed water in the Prussian Blue compound can be determined.
The temperature is then increased to 180°C, and drying continues while recording the mass until stabilization. This allows the calculation of the total crystalline water content and the determination of the crystalline water percentage.
This method is time-consuming and highly sensitive to environmental conditions. High ambient humidity can prevent complete water removal, compromising accuracy, and it is unsuitable for large-scale industrial testing.
Thermogravimetric analysis (TGA) determines the crystalline water content of Prussian Blue. During heating, water is released: initially, free water weakly bound via intermolecular forces evaporates, followed by more tightly bound crystalline water. Plotting mass loss against temperature allows quantification of water content.
Results are influenced by multiple factors such as atmosphere flow rate, sample packing, particle size, heating rate, and sample quantity. To obtain accurate measurements, these variables must be carefully controlled, requiring specialised expertise[3].
Therefore, an efficient, accurate, and straightforward method to measure crystalline water content in Prussian Blue compounds is urgently needed by sodium-ion battery manufacturers. Real-time, rapid detection of crystalline water is crucial for evaluating different processing techniques and product quality under varying conditions.

Low-field nuclear magnetic resonance (NMR) is an eco-friendly, rapid, non-destructive, and repeatable technique that probes materials at the proton level. It quantitatively characterises crystalline water in Prussian Blue compounds, offering a scientifically sound and convenient approach for rapid testing in sodium-ion batteries[4].
Low-field NMR testing
Before rapid, large-scale testing of Prussian Blue compounds using low-field NMR, a calibration curve linking NMR signal to water content is established. The calibration and testing results are as follows.
First, a measured mass of Prussian Blue compound is recorded and loaded into an NMR tube. The sample is then dried while continuously recording both the mass loss and the corresponding NMR signal.
The total water content in Prussian Blue is determined from the calibration curve. Using the ratio of crystalline to non-crystalline water NMR signals, the crystalline water content is quantified. Once the calibration is complete, large-scale, rapid testing can be conducted in under 2 minutes per sample.
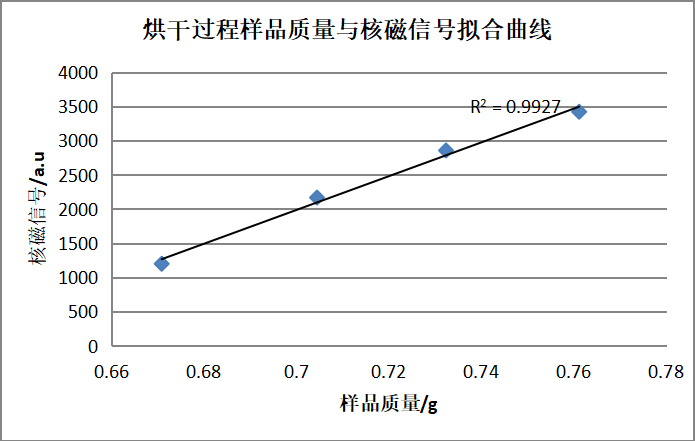
Figure: Sample mass vs. NMR signal during drying
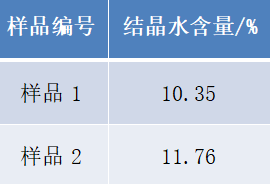
Test results
[1] Yuhao, Lu, Long, et al. Prussian blue: a new framework of electrode materials for sodium batteries[J]. Chemical Communications, 2012.
[2] Qi T, Jia M, Yuan Q, et al. Prussian blue analogs (PBA) derived heterostructure Zn-Fe bimetallic selenide nanospheres with high capacity sodium-ion storage[J]. Journal of Alloys and Compounds, 2023:940.
[3] Wang Z, Wu M, Chen G, et al. Co-pyrolysis characteristics of waste tire and maize stalk using TGA, FTIR and Py-GC/MS analysis[J]. Fuel, 2023, 337:127206-.
[4] Staltari G, Biasin A, Grassi L, et al. Rheological and Low Field NMR Characterisation of Cystic Fibrosis Patient’s Sputum[J]. Chemical and Biochemical Engineering Quarterly, 2023.
Low-field NMR testing
Before rapid, large-scale testing of Prussian Blue compounds using low-field NMR, a calibration curve linking NMR signal to water content is established. The calibration and testing results are as follows.
First, a measured mass of Prussian Blue compound is recorded and loaded into an NMR tube. The sample is then dried while continuously recording both the mass loss and the corresponding NMR signal.
The total water content in Prussian Blue is determined from the calibration curve. Using the ratio of crystalline to non-crystalline water NMR signals, the crystalline water content is quantified. Once the calibration is complete, large-scale, rapid testing can be conducted in under 2 minutes per sample.

Figure: Sample mass vs. NMR signal during drying

Test results
[1] Yuhao, Lu, Long, et al. Prussian blue: a new framework of electrode materials for sodium batteries[J]. Chemical Communications, 2012.
[2] Qi T, Jia M, Yuan Q, et al. Prussian blue analogs (PBA) derived heterostructure Zn-Fe bimetallic selenide nanospheres with high capacity sodium-ion storage[J]. Journal of Alloys and Compounds, 2023:940.
[3] Wang Z, Wu M, Chen G, et al. Co-pyrolysis characteristics of waste tire and maize stalk using TGA, FTIR and Py-GC/MS analysis[J]. Fuel, 2023, 337:127206-.
[4] Staltari G, Biasin A, Grassi L, et al. Rheological and Low Field NMR Characterisation of Cystic Fibrosis Patient’s Sputum[J]. Chemical and Biochemical Engineering Quarterly, 2023.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top