In 2021, the U.S. FDA issued a warning letter to a pharmaceutical company in Zhejiang. The firm was suspected of drug adulteration for failing to perform reliable verification testing on incoming APIs (active pharmaceutical ingredients) as required by 21 CFR 211.84(d)(1) and (2). The FDA demanded that the company “must commit to performing at least one specific identity test for each batch of incoming components.”
Many pharma companies use Raman spectroscopy for incoming material inspection. The 2020 edition of the Chinese Pharmacopoeia specifically notes: “Raman spectroscopy is suitable for chemical identification and rapid, non-destructive analysis of solid properties such as polymorphic transformations. It can also be applied in counterfeit detection and quality control.”
While Raman spectroscopy works well for small-molecule APIs and excipients, it is often ineffective for large molecules like therapeutic antibodies—leaving a gap in solutions. As a result, companies are forced to send antibody raw materials for traditional lab testing, such as SEC (size-exclusion chromatography) or CE (capillary electrophoresis). These methods consume time, manpower, and reagents. Using PMF (peptide mass fingerprinting) becomes an even larger undertaking, raising costs and delaying release of materials.
We therefore propose using low-field NMR technology for non-destructive identification of antibody raw materials upon receipt.

Low-field NMR has long been used in materials science and the food industry. Its principle lies in distinguishing hydrogen protons in a sample via NMR signals for qualitative analysis, or using the linear relationship between signal intensity and proton quantity for quantitative measurement. Examples include GB/T 6504-2008 Method for Determination of Oil Content in Chemical Fibres and GB/T 31743-2015 Determination of Solid Fat Content in Fats and Oils by Pulsed NMR.
In pharmaceuticals, low-field NMR is used for determining moisture content and distribution in drugs, qualitative and quantitative analysis of polymorphs, as well as in preclinical research for small-animal imaging and body-fat measurements.
Antibodies can be tested directly without opening the packaging—simply place them into the sample tube.

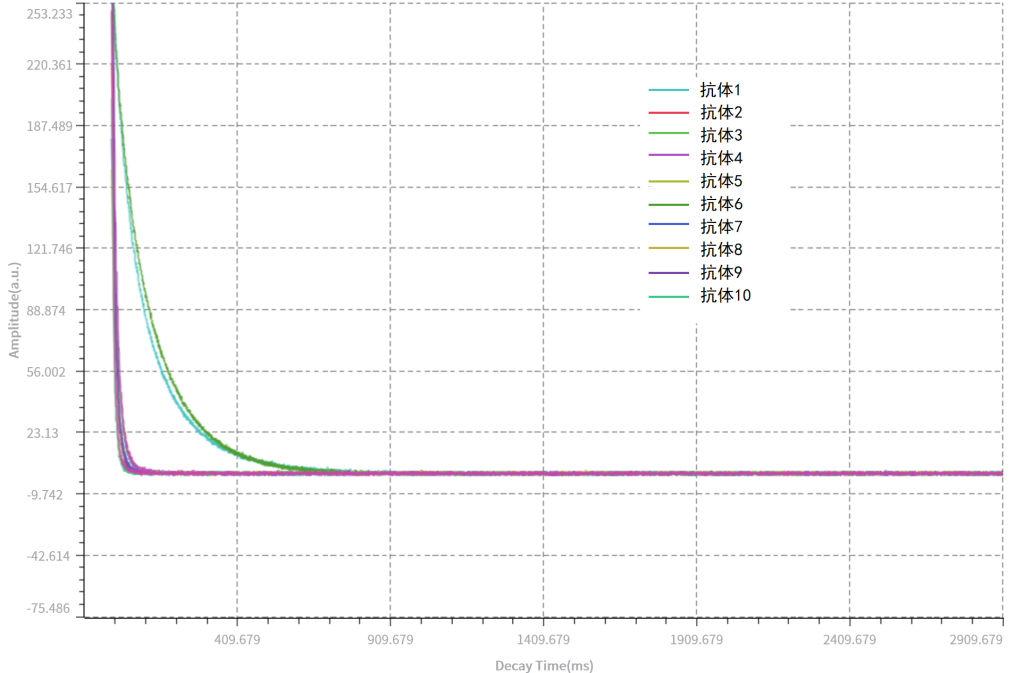
By measuring T2 relaxation times for 10 monoclonal antibody samples across two batches, samples can be rapidly screened and categorised, clearly differentiating between antibodies.
The entire process requires no pre-treatment, is completely non-invasive, and all samples can be tested within just half an hour.
Low-field NMR enables non-destructive identification of antibodies, preventing loss of scarce and expensive raw materials. Results show that relaxation analysis effectively differentiates antibody samples. With reference standards, it could further enable quantification and serve for quality control. In short, low-field NMR offers a compliant solution for antibody material receipt inspections and can be expanded to broader pharmaceutical applications.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top