The recycling of plastic waste is a pressing environmental challenge. Currently, mainstream methods such as mechanical recycling and incineration suffer from significant drawbacks—namely, material degradation and secondary pollution risks. Thermocatalytic pyrolysis offers a highly promising alternative by converting plastic waste into valuable base chemicals, balancing both environmental and economic benefits. Zeolite catalysts, with their unique porous structures and acidic active sites, have demonstrated excellent performance in catalytic pyrolysis reactions. However, traditional microporous zeolites face mass-transfer limitations, making them less effective for processing bulky or sterically hindered molecules like polymers. Hierarchical pore structures help overcome these transport barriers by significantly enhancing the diffusion of both reactants and products. To characterise the pore connectivity of such porous catalyst materials, commonly used techniques include scanning electron microscopy (SEM), gas adsorption (BET), and small-angle X-ray scattering (SAXS). Yet each has limitations: SEM provides only surface morphology, BET shows pore size distribution but lacks insight into pore shape or connectivity, and SAXS requires well-dispersed and stable samples with sufficient thickness and scattering intensity. This study introduces low-field nuclear magnetic resonance (NMR) technology to evaluate pore connectivity in catalysts by analysing the relaxation behaviour of water molecules within the pore networks.
This application investigates the pore connectivity of nitric acid-treated HY zeolite (DA(HNO3 0.5 h)-Y) and untreated HY zeolite (H-Y). Samples were first fully saturated by soaking in deionized water for 72 hours, ensuring sufficient water molecule penetration into the internal pores. T1–T2 relaxation measurements were then performed. For comparative analysis, pure water was also tested under identical conditions.
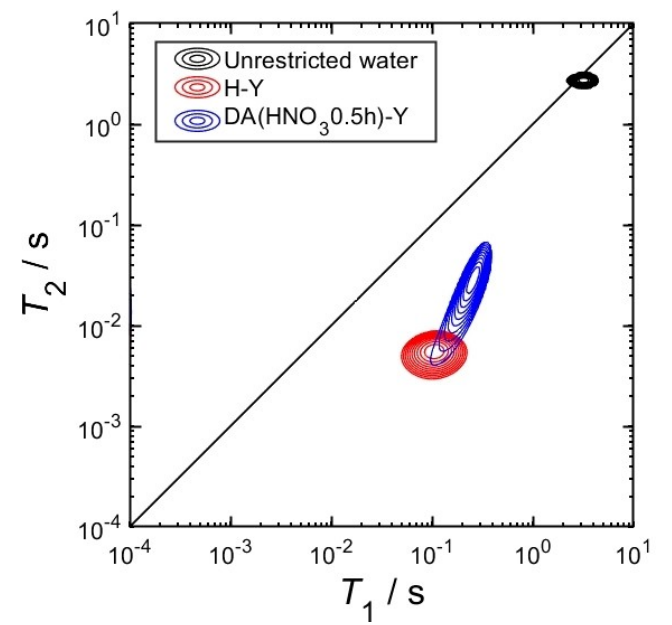
Figure 1: T1–T2 relaxation maps of free water, untreated H-Y zeolite, and nitric acid-treated H-Y zeolite (DA(HNO3 0.5 h)-Y)
For free water, a black relaxation population is observed in the map with T1 ≈ 3.1 s and T2 ≈ 2.7 s—characteristic of bulk liquid water. The untreated H-Y zeolite shows only a single (red) relaxation population, indicating rapid exchange of water molecules between micropores and inter-particle spaces—typical behaviour of water in constrained micropores. In contrast, the nitric acid-treated zeolite (DA(HNO3 0.5 h)-Y) also displays a single relaxation population, but with longer relaxation times and broader distribution. This suggests that water molecules are diffusing into larger pore structures and that the catalyst features more complex, interconnected pores—supporting the hypothesis of a hierarchical structure.
PGSTE (Pulsed Gradient Stimulated Echo) measurements were used to determine the self-diffusion coefficients (D) of water within the samples. For the untreated H-Y zeolite, signal attenuation data fitted well to a single exponential decay model, yielding a diffusion coefficient of D = 1.40 ± 0.02 × 10-9 m2/s. For the DA(HNO3 0.5 h)-Y zeolite, the signal attenuation fitted a bi-exponential model, identifying two distinct populations: – A dominant fast-diffusion population with D1 = 1.64 ± 0.02 × 10-9 m2/s, indicating long-range diffusion across micropores, mesopores, and interparticle spaces. – A minor slow-diffusion population with D2 = 3 ± 0.5 × 10-10 m2/s, likely representing restricted diffusion within blocked micropores.
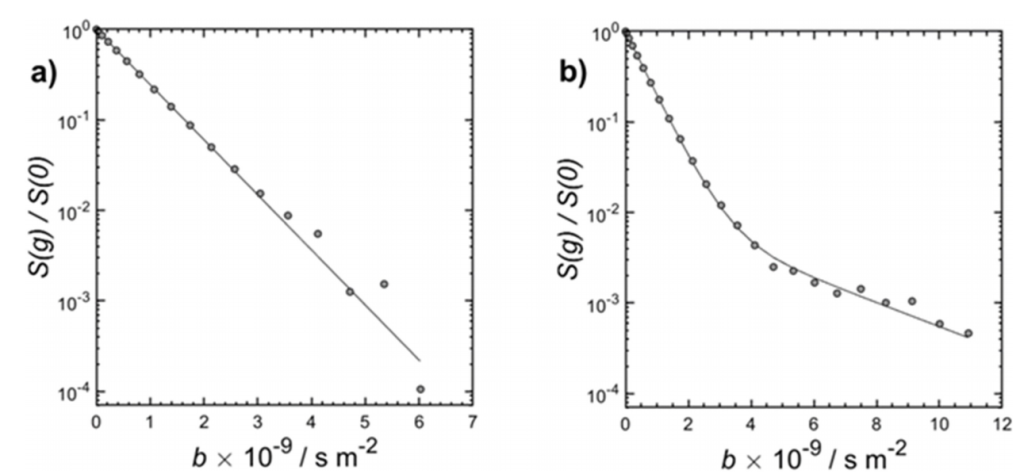
Figure 2: PGSTE measurement results — a: untreated H-Y zeolite (single exponential fit); b: nitric acid-treated H-Y zeolite (DA(HNO3 0.5 h)-Y) (bi-exponential fit)
This case study demonstrates the significant advantages of low-field NMR in characterising pore connectivity in catalysts, including: 1. Non-destructive testing without damaging sample structure 2. High sensitivity to detect subtle changes in microstructures 3. Simultaneous insights into both pore size and interconnectivity
It also provides a valuable reference for analysing pore architectures in other catalytic materials—supporting the development of next-generation, high-performance catalysts.
If you’re interested in this application, feel free to contact us: 15618037925
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top