To all friends following the forefront of food science, the “Niumag Cup” Jiangnan University Outstanding Case Study Series has reached its grand finale! The highlight research focuses on a core challenge in frozen food quality improvement — water migration and ice crystal damage. In this edition, we delve into the exciting study by the Jiangnan University team, which uses low-field Nuclear Magnetic Resonance (LF-NMR) technology to explore how magnetic field-assisted freezing regulates water migration in shrimp!
Freezing technology is key to extending the shelf life of aquatic products, such as shrimp, by slowing down biochemical processes and microbial growth to maintain quality. However, conventional freezing often produces large, irregular ice crystals due to insufficient freezing rates. These crystals severely damage tissue cells, trigger water migration, and lead to loss of flavour and nutritional value, affecting subsequent processing and consumer acceptance, thereby limiting product promotion and development. Therefore, controlling ice crystal size and reducing water migration is a central challenge in improving frozen seafood quality. For decades, optimisation focused on enhancing heat transfer efficiency. Yet, rapid freezing at very low temperatures is costly and can cause freeze cracking in some products. Recently, attention has turned to nucleation control technologies, aiming to accelerate freezing so that heat transfer outpaces water diffusion, forming uniformly distributed small intracellular ice crystals and protecting tissue. Among these, Magnetic Field-Assisted Freezing (MF) not only modifies ice nucleation but is environmentally friendly and energy-efficient. It does not alter internal pressure or generate oxidants during freezing, making it a novel green freezing technology.

Accurately characterising ice crystal formation and dynamic water distribution is critical for optimising freezing processes. Traditional methods often rely on destructive sampling, making real-time, in situ monitoring of water migration and ice crystal evolution difficult. In contrast, LF-NMR technology, with its non-destructive advantage, can effectively differentiate and quantify water in different states (e.g., free water, bound water) by analysing the relaxation properties of hydrogen protons, particularly T2 relaxation times, providing robust technical support to address these challenges.
1. Reveal the mechanism by which magnetic field-assisted freezing affects water migration in shrimp.
2. Optimise magnetic field intensity parameters to enhance water retention and texture in frozen shrimp.
Experimental Procedure:
Sample Preparation:
1. Pre-treatment of live shrimp;
2. Magnetic field-assisted freezing (0–80 mT);
3. LF-NMR and MRI measurements.
Key Parameters:
1. Magnetic field intensity gradient (20/40/60/80 mT);
2. Freezing temperature profile (pre-cooling, phase change, deep-freezing stages).
Analytical Techniques:
LF-NMR: Analyse water distribution via transverse relaxation time (T2);
MRI: Pseudocolour mapping to visually track water migration;
SEM/Optical Microscopy: Observe ice crystal morphology and muscle fibre damage.
The experiment used the NMI20-060H-I NMR imaging analyser (Niumag Analysis) for LF-NMR relaxation measurements. MRI results were visualised through multi-layer spin-echo pulse sequence imaging, directly showing water distribution in the samples.
1. Low-field NMR (LF-NMR) is a non-destructive method that analyses water distribution and states by measuring relaxation times of water molecules.
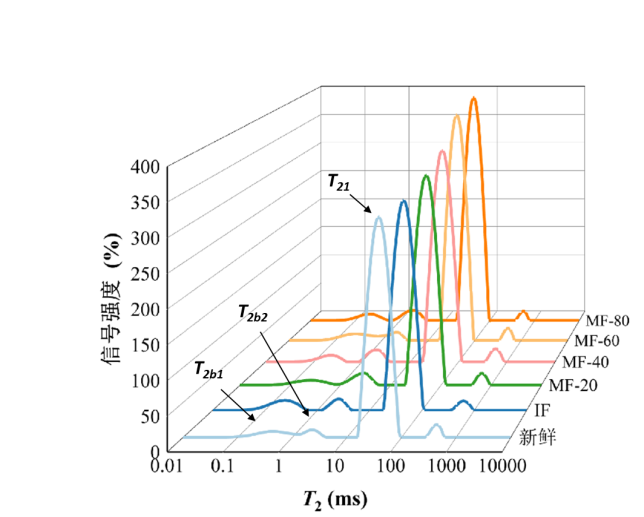
Figure 1: LF-NMR Curve
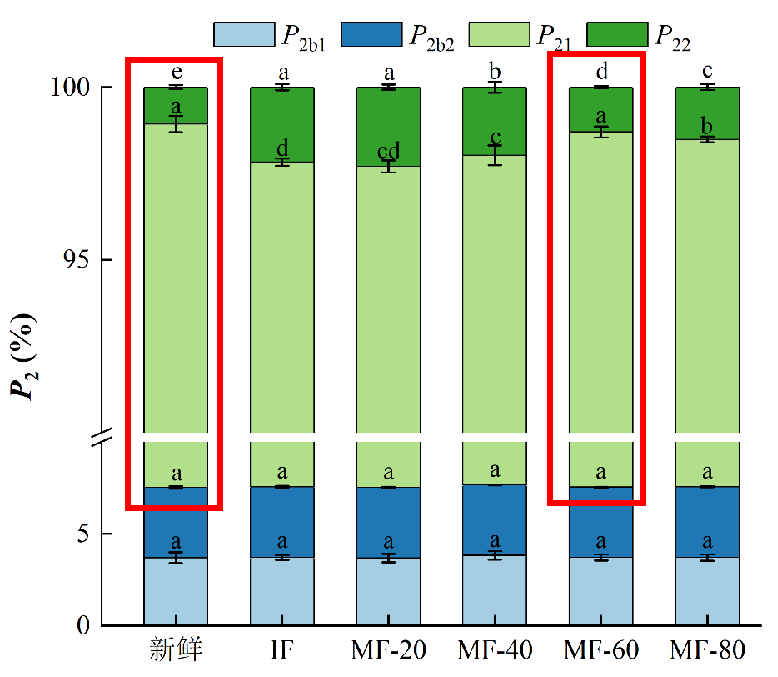
Figure 2: Relative content (%) of water fraction P2
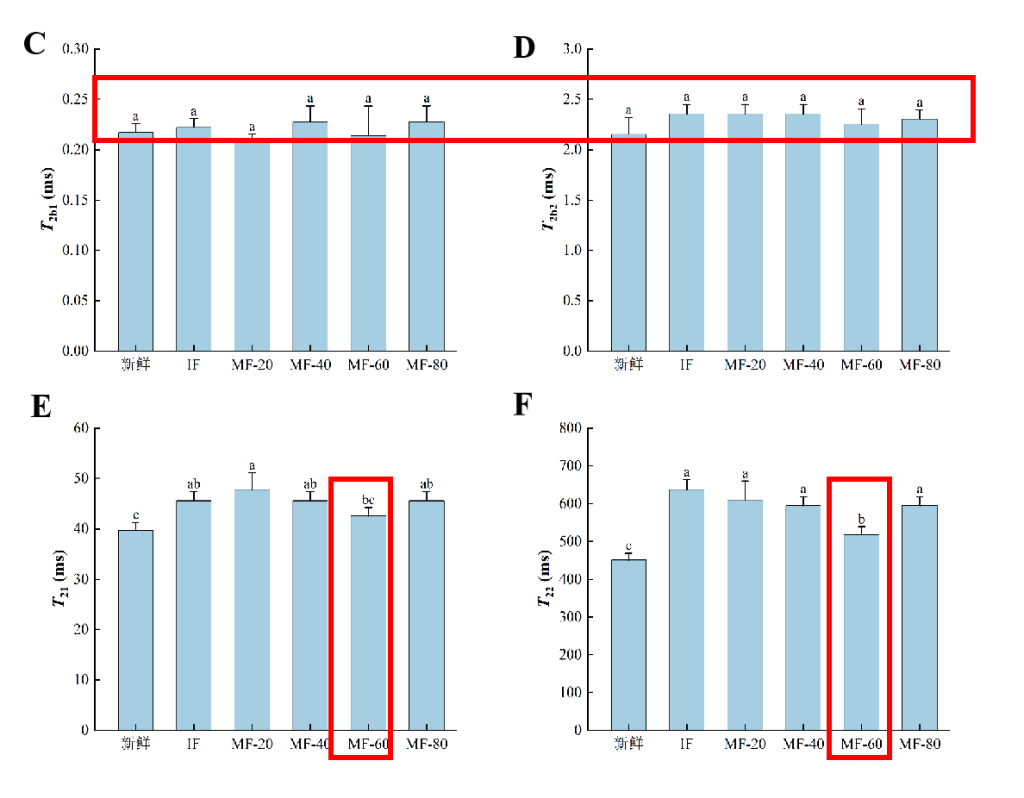
Figure 3: Transverse relaxation time T2 (ms) (IF: immersion freezing; MF: magnetic field-assisted freezing at varying intensities)
As shown, four distinct water populations were observed in the ranges 0–1 ms (T2b1), 1–10 ms (T2b2), 10–100 ms (T21), and 100–1000 ms (T22). T2b1 and T2b2 represent strongly and weakly bound water, respectively. T21 corresponds to immobilised water within dense myofibrillar networks, while T22</ sub> represents free water between fibre bundles, influenced by capillary forces. P2 indicates the relative water content of each group — higher P2 signifies higher water proportion. T2 reflects the sample’s water-binding capacity: lower T2 implies stronger binding, and vice versa.
1. Freezing increased T21 of immobilised water, suggesting weakened binding with other components and enhanced mobility. This may result from myofibrillar protein denaturation during freezing, altering hydration and partially breaking hydrogen and ionic bonds within the network.
2. The MF-60 treatment group showed lower T21 values than other frozen treatments, indicating that MF-60 effectively suppressed the increase in immobilised water mobility. This likely stems from MF-60 reducing large ice crystal formation, minimising damage to the myofibrillar network and limiting protein unfolding and denaturation.
2. Magnetic Resonance Imaging (MRI) is a rapid, non-invasive imaging technique that constructs internal water distribution maps by measuring proton relaxation times. Different colours in the images correspond to varying proton densities, reflecting regional water content within the sample.
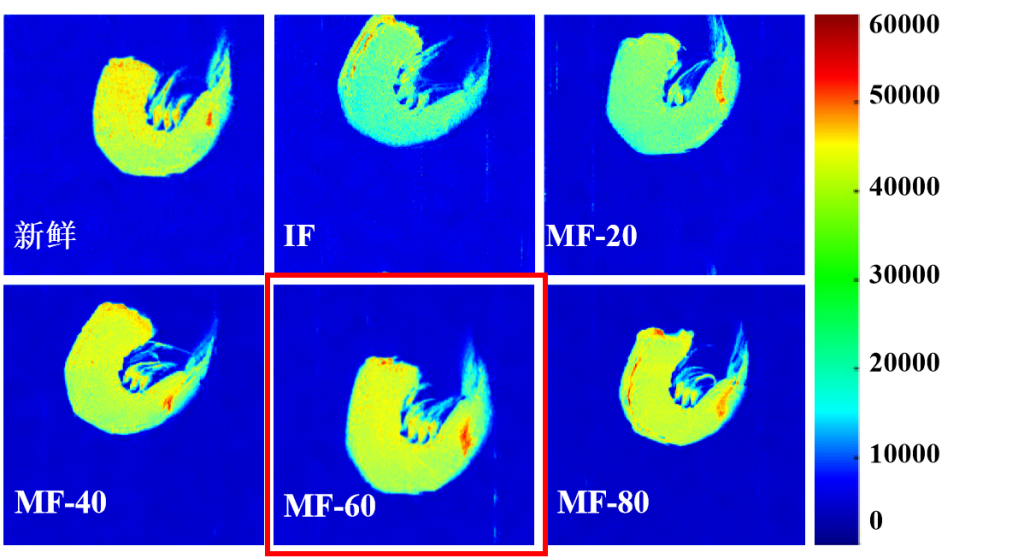
Figure 4: MRI images of frozen shrimp under different treatments; comparison with fresh shrimp
1. Fresh samples appear brightest red, indicating the highest water content.
2. Freezing darkens the image, reflecting water loss.
3. The MF-60 group shows brighter images than other frozen treatments, demonstrating that MF-60 effectively reduces water loss and enhances muscle water retention.
Experiments confirmed that static magnetic field-assisted freezing (MF) can significantly improve the frozen quality of Litopenaeus vannamei, with 60 mT providing the optimal effect. This treatment shortens freezing time, minimises ice crystal damage to muscle tissue, and improves water retention, texture, and appearance of frozen shrimp.
The “Niumag Cup” Jiangnan University Outstanding Case Study Series concludes with this finale! We thank the faculty and students of Jiangnan University for their exceptional contributions and every reader for your continued interest.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top