Still burning the midnight oil over massive piles of literature? Today’s AI tools, with intelligent parsing and multimodal analysis, can help you quickly pinpoint the key information you need.

This article provides a systematic review of targeted drug delivery (TDD) and integrated theranostic strategies for malignant lymphoma, with a focus on innovative applications of antibody–drug conjugates (ADCs), liposomes, and nanomedicines. In the section on MRI-based diagnostic and therapeutic strategies (Section 6), the authors highlight the following core topics:
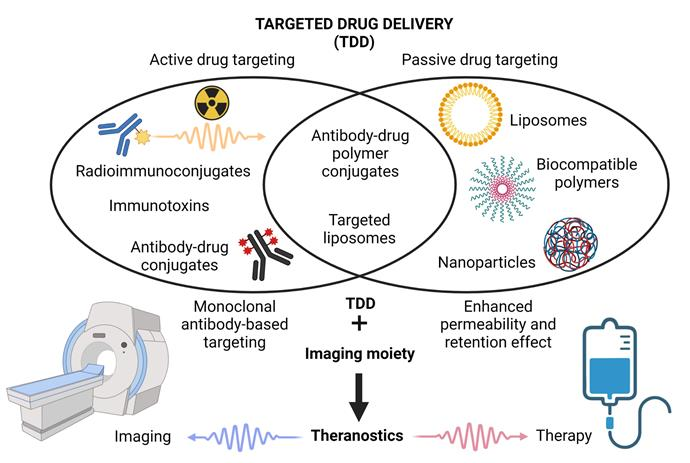
Gd-based contrast agents: As traditional T1-weighted imaging agents, gadolinium chelates enhance MRI signals by shortening longitudinal relaxation times. However, their theranostic applications remain largely confined to preclinical studies.
Manganese oxide nanoparticles (MONs): Offering low toxicity, high biocompatibility, and strong T1-enhancement, MONs can enable tumour microenvironment–responsive drug release (e.g., triggered by pH or reactive oxygen species), while simultaneously supporting imaging and therapy. For example, MONs loaded with chemotherapeutics allow real-time MRI monitoring of drug distribution and leverage the redox properties of manganese ions to induce apoptosis in tumour cells.
SPIONs passively target tumours via their superparamagnetism and the enhanced permeability and retention (EPR) effect, while also enabling active targeting through external magnetic field guidance. Their theranostic potential is threefold:
Magnetic hyperthermia: Tumour cell ablation through heat generated under alternating magnetic fields;
Drug delivery: Functioning as carriers for chemotherapeutic or immunotherapeutic agents;
Multimodal imaging: Serving as T2 contrast agents for MRI, and when combined with optical or nuclear imaging, significantly improving diagnostic accuracy.
Technical bottlenecks: Limited targeting efficiency of nanoparticles, complex in vivo clearance mechanisms, and potential toxicities (e.g., gadolinium deposition risk);
Clinical translation: Requires optimisation of nanoparticle stability, scalable manufacturing processes, and improved tumour specificity through combined targeting ligands (e.g., antibodies, aptamers);
Innovative pathways: Development of pH- or enzyme-responsive “smart” nanosystems, empowered by AI-assisted design, to achieve precise theranostics.
MRI-driven theranostic strategies, powered by nanotechnology, open new avenues for precision treatment of lymphoma. However, their clinical application is still constrained by challenges in targeting efficiency and safety. Future progress will rely on material innovation and cross-disciplinary collaboration to accelerate the transition of theranostic nanomedicines from laboratory research to clinical practice.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top