The oral route is the most common form of drug administration. With the use of combinatorial chemistry and high-throughput screening, the number of active pharmaceutical ingredients (APIs) with poor water solubility has been steadily increasing. The solubility of APIs in water is critical to their dissolution rate in the gastrointestinal tract. Poorly soluble APIs show low dissolution in gastric and intestinal fluids, significantly limiting oral absorption and resulting in low bioavailability.
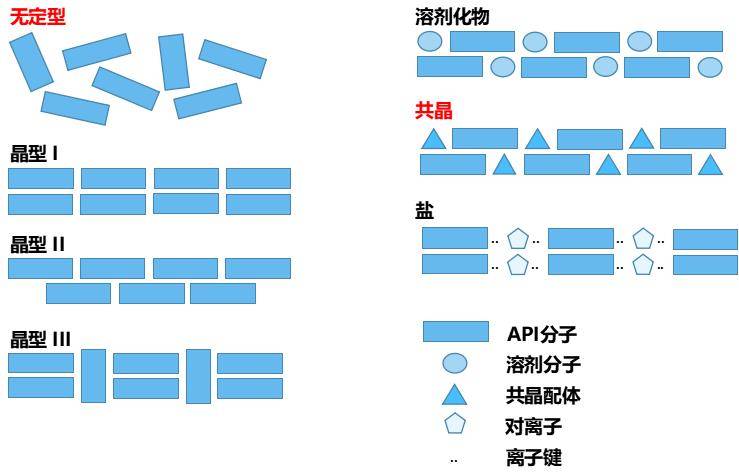
During formulation development, a range of techniques have been explored to improve solubility. These include altering the crystal form (polymorphism, co-crystals), particle-size reduction, and amorphisation through solid-dispersion technologies.
Among these, amorphisation via solid dispersion is regarded as a promising pharmaceutical approach to enhance solubility. Numerous studies have demonstrated significant improvements in dissolution. However, maintaining the amorphous state throughout the shelf life remains a major challenge. As such, detailed evaluation of the crystallinity of APIs in formulations is essential.
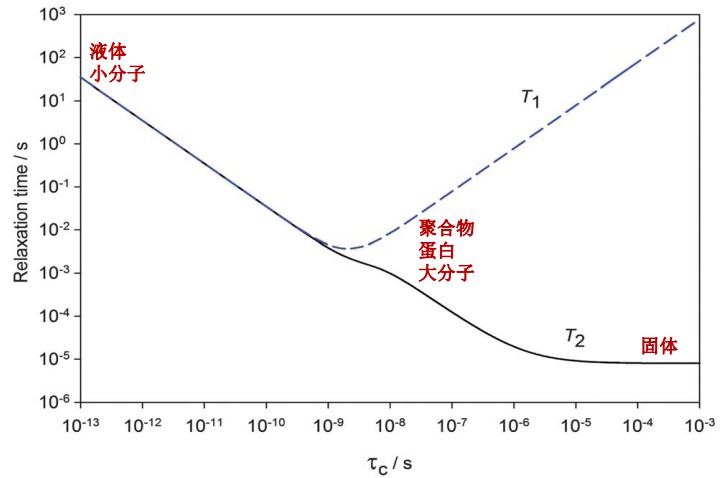
Low-field nuclear magnetic resonance (LF-NMR) is a benchtop analytical technology for measuring 1H relaxation. It can be used for both solid and liquid samples, providing fast, convenient measurements of T1 and T2 relaxation times. LF-NMR has been widely applied across scientific fields, including chemistry, food science, and materials research.
LF-NMR is also highly effective for distinguishing between crystalline and amorphous forms of APIs. By analysing relaxation parameters such as T1 and T2, differences in crystallinity can be identified. It serves as a complementary tool to traditional PXRD for evaluating crystallinity, with the advantages of short test times and simplified operation.
Experimental results show marked differences between crystalline and amorphous APIs in T1 relaxation behaviour. Crystalline APIs display longer T1 values than amorphous ones. Since relaxation times reflect molecular mobility through their relationship with rotational correlation times, lower molecular mobility in the solid state typically results in longer T1. These observations make LF-NMR a powerful method for assessing the crystallinity of API powders.
When APIs are thoroughly mixed with PVP at the nanoscale to form homogeneous solid dispersions, the T1 values differ significantly from those of the original amorphous API and PVP. This indicates that the molecular mobility of the amorphous API is substantially affected by interactions with PVP. LF-NMR can therefore assess component compatibility and molecular interactions within formulations.
By monitoring T1 relaxation behaviour, polymorphic transformations within physical mixtures can also be tracked.
Recommended instrument: PQ001 NMR Analyser for API crystallinity and amorphous-state testing.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top