Myofibrillar proteins play a crucial role in meat processing. During heating, they undergo thermal denaturation and aggregation, forming structures essential to meat quality. These changes directly affect the sensory attributes of proteins, such as elasticity, juiciness, and mouthfeel. Thermal gelation contributes to fine texture, product shaping, and moisture retention. The water-holding capacity (WHC) of myofibrillar proteins is particularly important for the functional properties of meat and meat products. Enzymatic catalysis can be applied to improve protein functionality.
Low-field NMR relaxometry is a non-destructive method capable of characterising proton mobility and water distribution. It has been widely used to measure WHC in meat. Proton relaxation includes spin–lattice relaxation time (T1) and spin–spin relaxation time (T2). T1 reflects interactions between spins and their environment, while T2 reflects spin mobility.
T2 values are most commonly used for assessing WHC in meat and meat products. T2 distinguishes between free water that does not interact with solid particles or macromolecules, and less-mobile water such as crystalline water or chemically/physically bound water. Numerous studies confirm that LF-NMR relaxometry is a valuable tool for studying myofibrillar proteins under varying pH, ionic strength, and heat treatments. Here, LF-NMR relaxometry is introduced for investigating enzymatic effects on myofibrillar protein gels.
Gelation combined with enzymatic crosslinking causes myofibrillar protein chains to bind, forming a continuous three-dimensional network in which water is entrapped. WHC reflects the protein’s ability to retain water and is widely used as an objective indicator of meat quality and yield. As shown below, increasing MTG from 0 to 2 U/g protein significantly raised the WHC of PMP gels from 81.4% to 95.6%. Beyond 2 U/g, up to 8 U/g protein, WHC did not increase further.
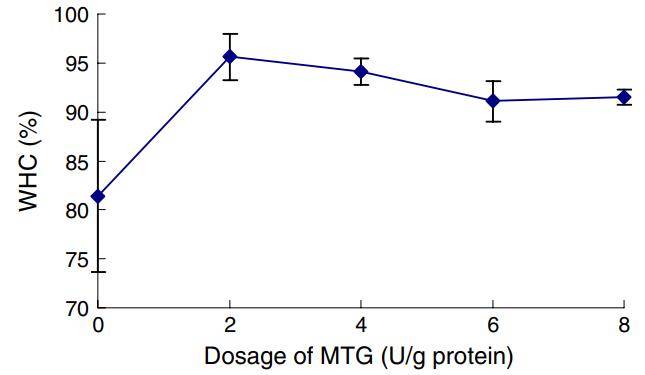
Water-holding capacity of myofibrillar protein gels at different enzyme concentrations
The T2 signal can be fitted to a distribution. The figure below shows T2 relaxation distributions of samples at different enzyme concentrations. As MTG concentration increases, the main peak clearly shifts to shorter relaxation times. At 2 U/g protein, the T2 peak broadens. However, at an incubation temperature of 50 °C, increasing MTG did not produce further peak changes.
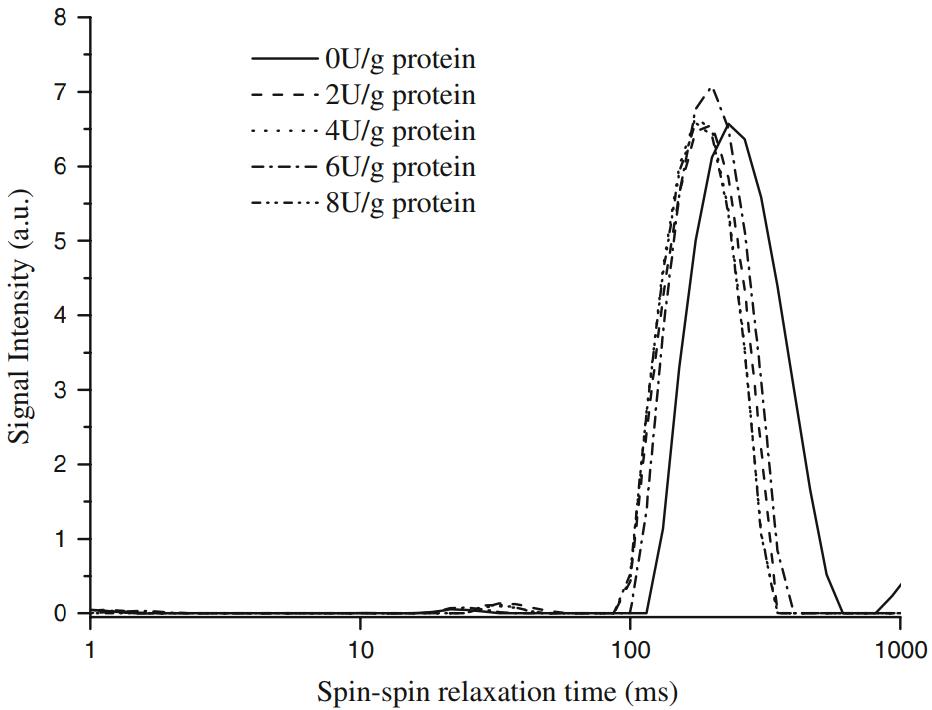
T2 relaxation distributions of myofibrillar protein gels under different enzyme concentrations
After enzyme addition, significant shifts in T2 relaxation times were observed. T2 decreased from 226 ms in the control group to 188 ms at 2 U/g protein. However, further increases in enzyme concentration up to 8 U/g protein produced no additional reduction.
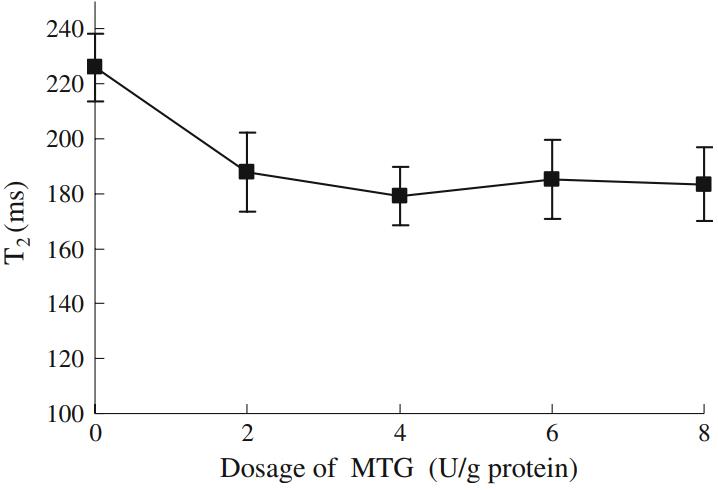
Effect of enzyme concentration on T2 relaxation times
LF-NMR results showed that T2 relaxation times shortened with enzyme addition, indicating reduced proton mobility due to network formation.
SEM observations confirmed that enzyme addition created a more porous microstructure in the myofibrillar protein gels. This structural change corresponded to reduced T2 and higher WHC.
Recommended instrument: NMI20-040V-I NMR Imaging Analyser

[Reference: Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. European Food Research & Technology, 2009, 228(4):665–670.]
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top