To investigate the evolution of pore structures during ionic rare earth leaching and to analyse how ion-exchange percolation affects the mineral’s microstructure, a saturated leaching experiment was designed using reconstituted rare earth samples.
Using low-field nuclear magnetic resonance (NMR) detection and imaging techniques, T2 spectra of the pore structures were obtained for leaching with H2O and (NH4)2SO4 solutions, from which pore distribution images were reconstructed.
Comparative analysis indicates that the factors influencing the microstructure of rare earth ores during leaching include both solution percolation and ion-exchange. Under their combined action, the dominant factor controlling pore distribution is the ion-exchange process.
Simple solution percolation increases pore size, transitioning the structure from small-to-medium pores toward larger pores. In contrast, ion exchange triggers particle movement and reorganisation, reducing pore size and shifting the structure from loose to compact.
Throughout the leaching process, ion-exchange progresses in layers along the flow direction, with chemical replacement and physical percolation alternately shaping the microstructure of the mineral.
Selected Experimental Results
NMR T2 Spectrum Analysis
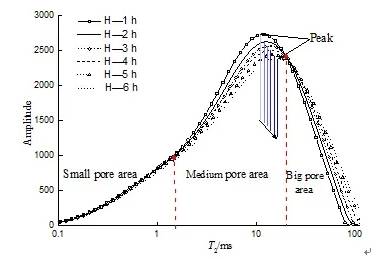
Figure 3 Pure water leaching stage (Stage 1)
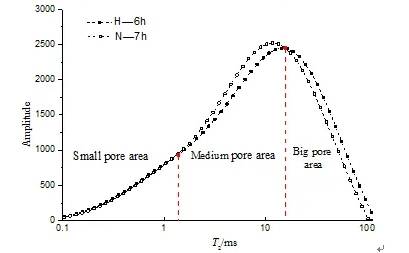
Figure 4 Transition stage: pure water to ammonium sulfate (Stage 2)
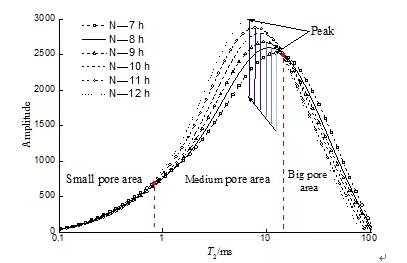
Figure 5 Ammonium sulfate leaching stage (Stage 3)
NMR Imaging Analysis
Extensive literature shows that the early pore structure of cement paste comprises cement particles of varying sizes, hydration products of different scales (including ettringite, calcium hydroxide, and C-S-H gel), and pores of multiple scales. These conditions correspond to fractal-like characteristics defined in fractal theory. In this study, pore structures of early cement paste at different water-to-cement ratios were investigated based on fractal theory. The results reveal scale-dependent fractal features of the pore network, with different fractal dimensions observed across distinct T2 ranges, as illustrated below.
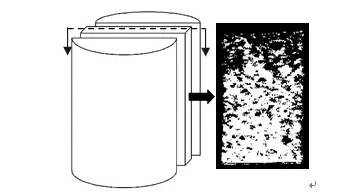
Figure 6 Reconstructed cross-sectional image of the sample
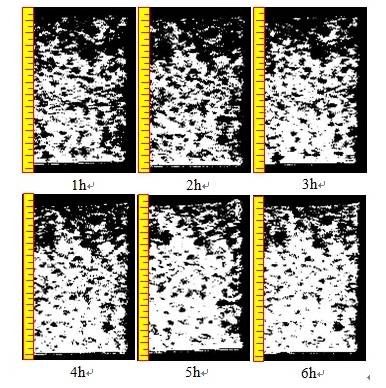
Figure 7 Reconstructed pore structure image during pure water leaching
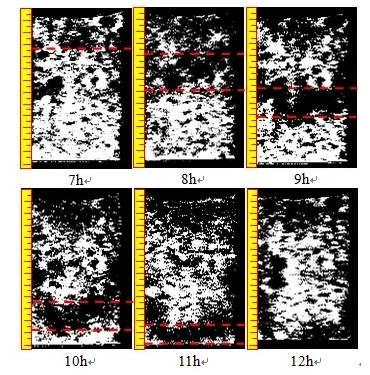
Figure 8 Reconstructed pore structure image during ammonium sulfate leaching
1. During the leaching process, the microstructure of the rare earth ore is governed by the coupled effects of solution percolation and ion exchange. Solution percolation increases pore quantity and size, resulting in a looser microstructure, whereas ion exchange causes particle rearrangement, reducing pore size and quantity, and creating a denser structure.
2. The ion-exchange reactions progress layer by layer along the solution flow, consistent with laminar flow theory. Under the combined effect of percolation and ion exchange, the primary influence on microstructure arises from the chemical action of ion exchange rather than the physical effects of percolation. Overall, the chemical replacement reaction during ionic rare earth leaching contributes to microstructural refinement of the mineral.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top