Water-dispersible granules (WDGs) are granular formulations that rapidly disintegrate and disperse into a suspension when mixed with water. In the U.S., they are often referred to as dry flowables or dry suspensions. Strictly speaking, there is a distinction: WDGs are obtained by dry milling and granulating pesticides (using methods such as pan granulation, extrusion, fluidized bed agglomeration, etc.), followed by drying. In contrast, dry suspensions are produced by wet milling the pesticide into a suspension, then drying and granulating via spray drying to obtain solid microparticles.
WDGs emerged in the 1980s as a new pesticide formulation. Due to advantages like good safety, no organic solvents, dust-free handling, and ease of packaging, transport, and application, they gained rapid popularity in the agrochemical market.
The development of pesticide formulations is closely linked to their dispersion behavior. An effective pesticide, an appropriate formulation, and the right application equipment all depend on good dispersion properties. There are two main strategies to improve dispersion: (1) Processing — reducing particle size through grinding increases dispersion. For instance, early dust formulations (powder pesticides) were made by mixing active ingredients, carriers, and adjuvants. While easy to use, cost-effective, and efficient in coverage, they created dust pollution, posed health hazards, and had low dispersion, leading to their decline in favor of more advanced formulations with better dispersion.
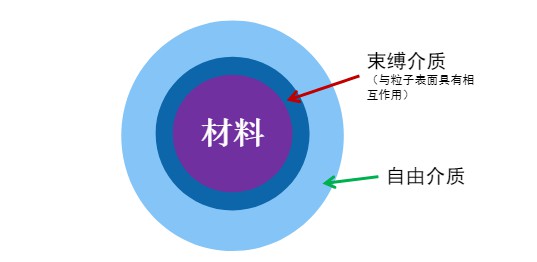
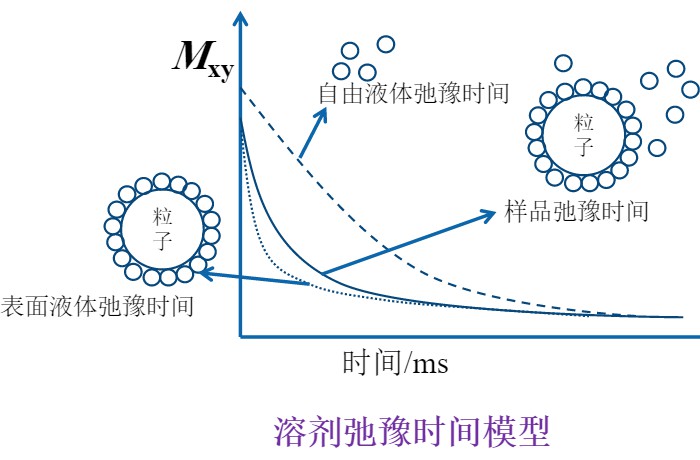
Low-field nuclear magnetic resonance (LF-NMR) technology can be used to evaluate the dispersion performance of pesticide WDGs. It enables rapid analysis of particle dispersion, agglomeration, and flocculation behavior in suspension systems. This provides valuable reference data for WDG pesticide formulation development and quality control.

In particle dispersions, the relaxation rate of the solvent is linearly related to the available particle surface area. Solvents associated with free polymers or within polymer loops/tails show little change in relaxation rate due to their high mobility. However, when polymers adsorb onto particle surfaces, the local water molecule content and/or residence time near the surface increases, resulting in a higher overall relaxation rate. This difference in NMR relaxation allows LF-NMR to quantitatively evaluate particle dispersion behavior.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top